Conditions
Multiple Sclerosis
Multiple sclerosis (MS) is the most common autoimmune disease affecting the central nervous system. In MS, the immune system attacks the myelin sheath, a protective material around nerves in a process called demyelination. Damage to the myelin sheath disrupts the transmission of information within the brain and between the brain and the body resulting in various neurological symptoms.
The most common symptoms of MS include fatigue, weakness, numbness and tingling, blurred vision, double vision, poor coordination and balance, and others. Tremor, paralysis and blindness are less common symptoms of MS.
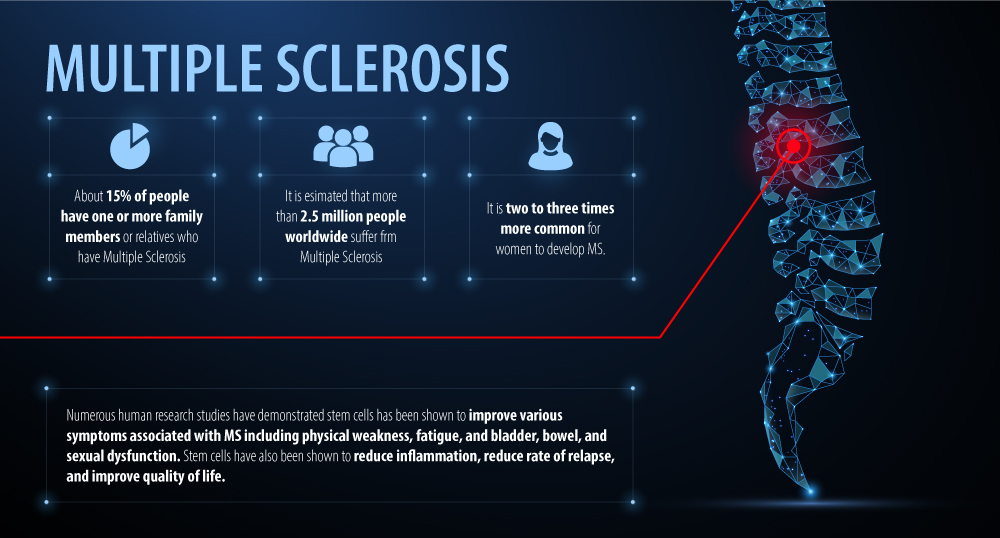
It is estimated that more than 2 million people have MS worldwide. Although anyone can develop MS, it is 2-3 times more common in women and overall more common in Caucasians of northern European ancestry.
MS is characterized by demyelination, progressive neurological dysfunction, and episodes of remission and relapse. However, MS is an unpredictable disease with different clinical presentations and as such four subtypes of MS have been defined. They include clinically isolated syndrome, relapsing-remitting MS, secondary progressive MS and primary progressive MS.
There is currently no cure and no proven therapies to stop or reverse the progressive phases of the disease. The conventional approach includes medications, physical therapy, exercise therapy and others. Although numerous FDA approved drugs exist for MS, their impact is limited and appear to be effective at reducing the inflammatory aspect of the disease and reducing rate of relapse but do not appear to limit degeneration of the central nervous system. Evidence exists to support a reparative role of stem cell therapy to suppress inflammation and rebuild the injured nervous system in demyelinating diseases such as MS.
Numerous research studies have demonstrated that intravenous stem cell therapy is safe and effective in the treatment of MS. Stem cell therapy has been shown to improve various symptoms associated with MS, reduce rate of relapse and improve quality of life.
Based on early human clinical trials there appears to be a strong argument for the safe and effective use of intravenous stem cell therapy in the treatment of MS. While we wait for phase II/III trials the current data is encouraging and supports use of stem cell therapy in the clinical setting.
| Year | Author | No. of Patients | Delivery and Cell Type | Follow-up | Safety Outcome | Efficacy Outcome |
| 2018 | Riordan et al | 20 | IV UC-MSCs | 12 months | No SAEs and no deaths occurred. No AEs were classified as definitely related to study treatment. 6 moderate and 66 mild AEs. The most common AEs were headache and fatigue. | Improvements in bladder, bowel and sexual function. Improvements in physical function, energy and fatigue. Multiple assessment tools showed improvement after treatment. 83% showed no disease progression on MRI after 1 year. |
| 2018 | Meng et al | 2 | IV UC-MSCs | 8 years | No toxic reaction detected over 8 year follow up. All physiological examinations revealed normal indexes. Adverse reactions included fever, dizziness, headache, skin redness and vascular irritation which mainly occurred during infusion. Fever was the most common adverse reaction which resolved without medical intervention within 36 hours. | Reduced number of brain lesion foci on MRI. Improved and stabilized EDDS scores in 2 patients. Reduced inflammatory cytokines correlated with condition stabilization. The mean annual frequency rate of attacks was remarkably reduced compared to before baseline. |
| 2018 | Fernandez et al | 30 | IV AD-MSCs | 12 months | No related SAEs occurred and laboratory tests, vital signs, and spirometry did not identify any safety issues. A total of 70 AEs were reported during the trial period. The most frequent AEs were urinary tract infections, respiratory infection and anemia. Four serious AEs were reported and none of them were considered to be related to treatment. | No clear effects from stem cell therapy in any of the measurements used to assess efficacy, however this study was not powered to determined efficacy. MRI showed non-statistically significant changes in the number of active lesions compared to baseline. Improvement trends in evoked potentials in legs and eyes and active lesions on MRI. |
| 2018 | Cohen et al | 24 | IV BM-MSCs | 12 months | No severe or serious adverse events related to stem cell therapy. No patients developed clinical or laboratory indications of autoimmune phenomena and none had clinical or radiological evidence of paradoxical disease activation of MS. | No further activation or inhibition of new/enlarged brain lesions. 75% of patients were free of relapse during the post-infusion follow up period. EDSS scores of most patients were improved from baseline. Various exploratory efficacy outcomes were evaluated and were generally stable over the course of the study. |
| 2014 | Li et al | 23 | IV UC-MSCs | 12 months | The clinical safety was demonstrated without any reported significant adverse effects during 12 month follow up. No SAEs reported. | Stem cell therapy showed significant improvement in EDSS. More steady disease course and fewer incidences of relapse and significant change in anti-inflammatory cytokines in treatment group. |
*IV = intravenous, UC-MSCs = umbilical cord derived mesenchymal stem cells, AD-MSCS = adipose derived mesenchymal stem cells, BM-MSCs = bone marrow derived mesenchymal stem cells, AEs= adverse events, SAEs = serious adverse events, EDDS = expanded disability status scale
Early clinical studies of stem cells in multiple sclerosis:
- Improvement in bladder, bowel, and sexual dysfunction (Riordan et al)
- Improvement in walking (Riordan et al, Cohen et al)
- Improvement in upper extremity physical function, energy and fatigue (Riordan et al)
- Improvement in neurological status measured by Expanded Disability Scale Score (EDSS) (Riordan et al, Meng et al, Cohen et al, Li et al)
- Improvement in quality of life (Riordan et al)
- Reduction in number of brain lesions (Meng et al, Llufriu et al)
- Reduction in average number of relapses (Meng et al, Li et al)
Specific Conclusions From Selected Studies:
“We have shown that the intravenous infusion of UCMSC over several days is safe in subjects with MS. Additionally, UCMSC infusions may hold benefits, since this small study group saw improvement in bladder, bowel, and sexual dysfunction, walking, upper extremity physical function, energy and fatigue, general perspective of a positive health change and improved quality of life, and MRI lesions.”
- Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. (Riordan et al, 2018)
“Our findings confirm that UCMSCs have function of immune regulation and nerve protection, indicating the feasibility of UCMSC transplantation for multiple sclerosis.”
- Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. (Meng et al, 2018)
“The present study demonstrates that infusion of AdMSCs is a safe and feasible procedure in patients with SPMS. Although the study was not powered to determine the efficacy, some hint of efficacy was observed by the use of MRI and evoked potentials.”
- Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. (Fernandez et al, 2018)
“Our phase I study results support the feasibility, safety, and tolerability of IV administration of autologous, culture-expanded, bone-marrow-derived MSCs in MS.”
- Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. (Cohen et al, 2018)
“During a 1-year observation, no significant adverse effects were found, indicating the clinical safety could be well accepted. The significantly lower relapse occurrence and EDSS scores were found in the experimental group compared to the control group.” “Our study contributed, in part, to providing more evidence for the potential of hUC-MSCs as a therapy for MS.”
- The Potential of Human Umbilical Cord-Derived Mesenchymal Stem Cells as a Novel Cellular Therapy for Multiple Sclerosis. (Li et al, 2014)
“Bone-marrow-MSCs are safe and may reduce inflammatory MRI parameters supporting their immunomodulatory properties.”
- Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. (Llufriu et al, 2014)
For additional information and details regarding these studies please visit our Research Archive.
Exosomes have potential significant therapeutic effects in regenerative medicine, anti-aging and chronic disease. Click here to learn more about Exosome Therapy.
Brought to you by:
Ahvie Herskowitz, MD, President of ACAM
Director of Anatara Medicine
Clinical Professor of Medicine at UC San Francisco (2014)
(Read Dr. Herskowitz’s Bio Here)
Disclaimer
The contents of Understanding Stem Cells, such as text, graphics, images and other materials are for educational purposes only. The content is not intended to be a substitute for professional medical advice, diagnosis or treatment. You are encouraged to confirm any information on this website with other sources and review all information regarding any medical condition or treatment with your physician.
View our Terms and Conditions

