Conditions
Stroke
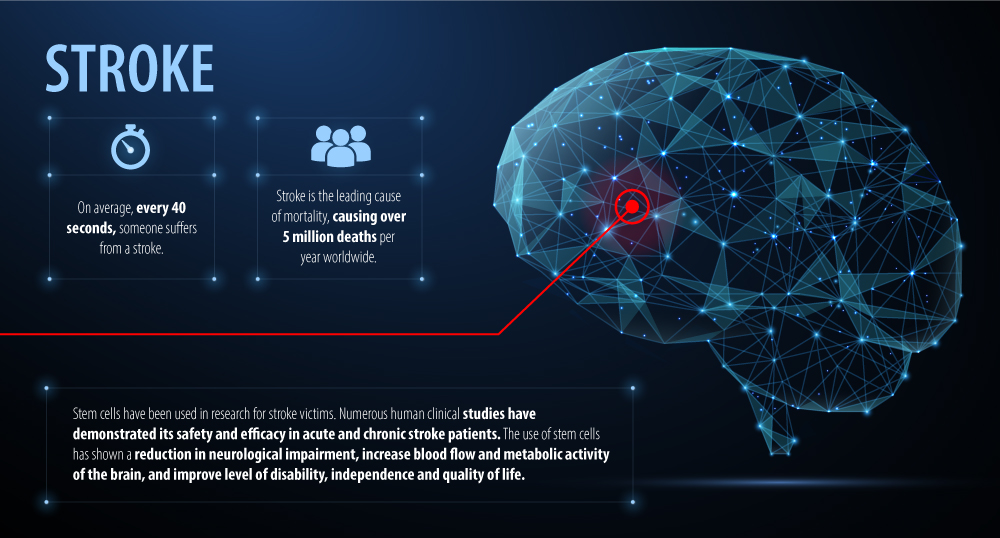
Stroke is a leading cause of mortality and is estimated to cause 5 million deaths per year worldwide. On average an American will suffer a stroke every 40 seconds and every year approximately 795,000 Americans have a new or recurrent stroke. Currently, there are limited conventional treatments for acute stroke such as intravenous tissue plasminogen activator which must be administered in a narrow window of 4.5 hours after the incident and even fewer treatment options for chronic stroke. Despite recent advancements in therapeutic options and rehabilitation strategies stroke remains a significant cause of disability.
Stem cell infusions have shown to be a promising novel therapy and is recognized in both acute and chronic stroke with human clinical studies demonstrating its safety and effectiveness. Stem cells from various tissue types have been investigated to treat stroke patients including bone marrow, adipose, and umbilical derived stem cells. The use of bone marrow derived stem cells has been the major focus within this area of research. However, due to the invasiveness of the procedure to harvest bone marrow stem cells there is growing interest in other cell types.
Common routes of administration used in stroke research include intravenous, intra-arterial (injected into arteries), intracerebral (surgically implanted into brain) and intrathecal (injected into the spinal canal). The advantage of intravenous injection is that it is less invasive and does not require sedation and/or surgery. Results from published research suggest that intravenous injection of stem cells is safe and effective.
Based on early human clinical trials there appears to be a strong argument for the safe and effective use of stem cell therapy in the treatment of stroke. While we wait for phase II/III trials the current data is encouraging and supports use of stem cell therapy in the clinical setting.
Early Human Clinical Trials of Stem Cells in Stroke:
- Significant improvement in various neurological assessment tools including National Institutes of Health Stroke Scale (NIHSS), Barthel Index (BI) and modified Rankin Scale (mRS) (Levy et al, Vahidy et al, Ouyang et al, Laskowtiz et al, Xue et al, Taguchi et al)
- Reduction in neurological impairment (Xue et al)
- Improvement in level of disability and independence (Xue et al)
- Increased structural integrity and coherence of axon fibers in the brain suggesting microstructural repair (Vahidy et al)
- Improved blood flow and increased metabolic activity of brain (Taguchi et al)
- Improved quality of life (Ouyang et al)
| Year | Author | No. of Patients | Delivery and Cell Type | Follow-up | Safety Outcome | Efficacy Outcome |
| 2019 | Levy et al | 21 | IV BM-itMSCS | 12 months | AEs were few during the 12 month follow up period. 15 SAEs were reported and none were ‘possibly’ or ‘probably’ related to treatment. Two mild AEs which included urinary tract infection and irritation at injection site were ‘possibly’ related to treatment. | Neurological assessment endpoints including the NIHSS and BI showed significant improvement. 35.5% of patients achieved excellent functional outcomes at 12 months. |
| 2019 | Vahidy et al | 25 | IV BM-MNCs | 24 months | All patients successfully completed the study with only 24 reported SAEs. None of the SAEs were deemed to be related to treatment. Of the 227 reported AEs, only 31 were deemed to be related to the study of which 28 were mild (Grade 1). | At 3 months, the treatment group showed significant difference in median mRS compared to historical control group. No significant change was observed in NIHSS at any time. FA was increased at 6 and 24 months compared to baseline demonstrating an increase in corticospinal tract fiber volume and myelin sheet thickness and is suggestive of microstructural repair. |
| 2018 | Laskowitz et al | 10 | IV UCB | 12 months | 113 AEs were reported in 10 participants; 112 were deemed ‘unrelated’ and 1 was deemed ‘possibly related’. UCB was safe and well tolerated by these ischemic stroke patients. | At 3 months all participants showed improved outcomes as measured by change in mRS and NIHSS. All showed improvement in ADLs as measured by BI. Normal evolution of the stroke with no significant increase in infarct volume. |
| 2017 | Hess et al | 129 | IV BM-MAPCs | 3 months | There were no dose-limiting toxicity events in either group. There were no infusion or allergic reactions and no difference in treatment-emergent adverse events between the groups. | At 3 month follow up there was no difference between treatment groups and placebo groups in global stroke recovery. |
| 2015 | Taguchi | 12 | IV BM-MNCs | 6 months | SAEs were observed in two patients over 6 month follow up; one had aspiration pneumonia and sepsis which was deemed not related to cell therapy, and the other had recurrent stroke which was deemed unclear if it was associated with cell therapy. No patients experienced death at discharge. | At 30 day follow up mean NIHSS score showed a statistically significant improvement by 4.8 +/- 4.6. Results indicate trends toward improved blood flow as measured by three parameters; regional cerebral blood flow (rCBF), and regional metabolic rate of oxygen consumption (rCMRO2) and oxygen extraction fraction (OEF). |
* IV = intravenous, AEs = adverse events, SAEs= serious adverse events, BM-itMSCs = bone marrow derived ischemic tolerant mesenchymal stem cells, BM-MAPCs = bone marrow derived multipotent adult progenitor cells, BM-MNCs = bone marrow derived mononuclear cells, NIHSS = National Institutes of Health Stroke Scale, BI = Barthel Index, mRS = modified Rankin Score, FA = fractional anisotropy, UCB = umbilical cord blood, ADL = activities of daily living
Specific Conclusions From Selected Studies:
“Intravenous transfusion of allogeneic ischemia-tolerant mesenchymal stem cell in patients with chronic stroke and substantial functional deficits was safe and suggested behavioral gains.”
- Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. (Levy et al, 2019)
“Bone marrow harvest and infusion of bone marrow derived stem cells is “safe and feasible in patients with acute ischemic stroke.”
- Intravenous Bone Marrow Mononuclear Cells for Acute Ischemic Stroke: Safety, Feasibility, and Effect Size from a Phase I Clinical Trial. (Vahidy et al, 2019)
“Stem cell transplantation can significantly improve neurological deficits and quality of life in patients with ischemic stroke, without severe adverse reactions.”
- Meta-Analysis of the Safety and Efficacy of Stem Cell Therapies for Ischemic Stroke in Preclinical and Clinical Studies. (Ouyang et al, 2019)
“UCB in adults after acute ischemic stroke is safe, well tolerated and feasible. In addition, improvements in functional outcomes were observed in all participants 3 months post-infusion.”
- Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke: Clinical Outcomes from a Phase 1 Safety Study. (Laskowitz et al, 2018)
“Our analysis verified the safety and efficacy of MSC therapy for ischemic stroke (IS). It significantly mitigated neurological defects and improved life quality of IS patients, without causing serious adverse events.”
- Mesenchymal stem cell transplantation as an effective treatment strategy for ischemic stroke in Asia: a meta-analysis of controlled trials. (Xue et al, 2018)
“Stem cell therapy with “multipotent adult progenitor cells were safe and well tolerated in patients with acute ischemic stroke.”
- Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. (Hess et al, 2017)
“Our study demonstrates that intravenous administration of autologous bone marrow mononuclear cells to patients with severe embolic stroke was feasible and safe. Positive results and trends favoring neurological recovery and improvement in cerebral blood flow and metabolism in post-stroke patients receiving therapy underscore the potential of this approach.”
- Intravenous Autologous Bone Marrow Mononuclear Cell Transplantation for Stroke: Phase1/2a Clinical Trial in a Homogeneous Group of Stroke Patients. (Taguchi et al, 2015)
For additional information and details regarding these studies please visit our Research Archive.
Exosomes have potential significant therapeutic effects in regenerative medicine, anti-aging and chronic disease. Click here to learn more about Exosome Therapy.
Brought to you by:
Ahvie Herskowitz, MD, President of ACAM
Director of Anatara Medicine
Clinical Professor of Medicine at UC San Francisco (2014)
(Read Dr. Herskowitz’s Bio Here)
Disclaimer
The contents of Understanding Stem Cells, such as text, graphics, images and other materials are for educational purposes only. The content is not intended to be a substitute for professional medical advice, diagnosis or treatment. You are encouraged to confirm any information on this website with other sources and review all information regarding any medical condition or treatment with your physician.
View our Terms and Conditions

